
What does osmosis and diffusion have in common? What is the ending result of diffusion and osmosis? Is osmosis considered active or passive transport?
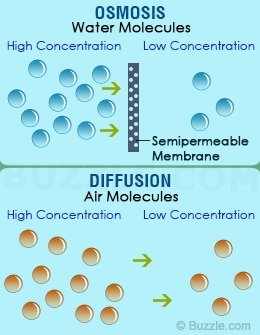
Both osmosis and diffusion equalize the concentration of two solutions. Both diffusion and osmosis are passive transport processes, which means they do not require any input of extra energy to occur. In both diffusion and osmosis , particles move from an area of higher concentration to one of lower concentration. Osmosis is the result of diffusion across a semipermeable membrane. If two solutions of different concentration are separated by a semipermeable membrane, then the solvent will tend to diffuse across the membrane from the less concentrated to the more concentrated solution.
Diffusion Diffusion refers to the process by which molecules intermingle as a result of their kinetic energy of random motion. Consider two containers of gas A and B separated by a partition. The molecules of both gases are in constant motion and make numerous collisions with the partition.
A simple rule to remember is: Salt is a solute, when it is concentrated inside or outside the cell, it will draw the water in its direction. Start studying Osmosis and Diffusion. Learn vocabulary, terms, and more with flashcards, games, and other study tools.
Theresa Knapp Holtzclaw. The processes of diffusion and osmosis account for much of the passive movement of molecules at the cellular level. In this laboratory, you will study some of the basic principles of molecular movement in solution and perform a series of activities to investigate these processes. The diffusion model of osmosis is rendered untenable by the fact that osmosis can drive water across a membrane toward a higher concentration of water.
The bound water model is refuted by the fact that osmosis is independent of the size of the solute molecules—a colligative property —or how hydrophilic they are. High Quality Membranes Made in the US. Cost Effective, Low Maintenance. Save Time By Searching Over 350Lessons!
Diffusion and Osmosis are physical processes that are often confused with one another and people find it difficult to differentiate between the two. These are processes that are found in nature and are related with movement of atoms and molecules from areas of higher concentration to areas of lower concentration. Diffusion and Osmosis The cell membrane plays the dual roles of protecting the living cell by acting as a barrier to the outside worl yet at the same time it must allow the passage of food and waste products into and out of the cell for metabolism to proceed. Test your knowledge on the processes of diffusion , osmosis , and tonicity!
In biology, both terms pertains to a type of passive transport. To be more detaile diffusion is a process by which molecules of a particular matter move from areas of high concentration to areas of low. Diffusion sees molecules in an area of high concentration move to areas with a lower concentration, while osmosis refers to the process by which water moves through a semipermeable membrane, leaving other bits of matter in its wake.
Learn and observe the concepts of diffusion and osmosis in the context of cell biology. Diffusion is when molecules or atoms move from an area of high concentration to an area of low concentration.
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.